 |
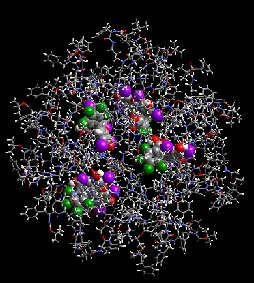
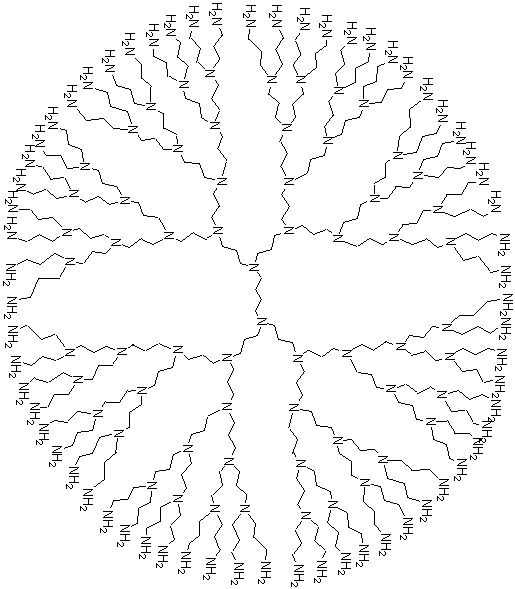
WHAT ARE DENDRIMERS?Dendrimers are branched molecules that center around a single molecule called the core. The word Dendrimer comes from the Greek word, Dendron, which means tree. There are two types of dendritic molecular species. They are separated according to their molecular weight (low and high). The low-molecular weight molecules are the dendrons and dendrimers while the high-molecular weight molecules are the brush-polymers, dendronized polymers and hyperbranched polymers. The low-molecular weight dendrons and dendrimers are macromolecules not polymers like the high-molecular weight brush-polmers etc. |
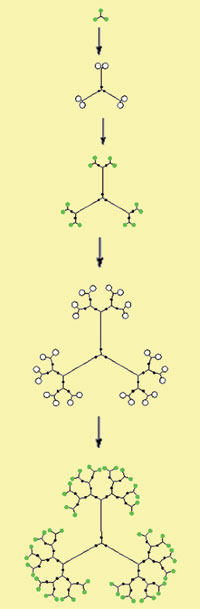 |
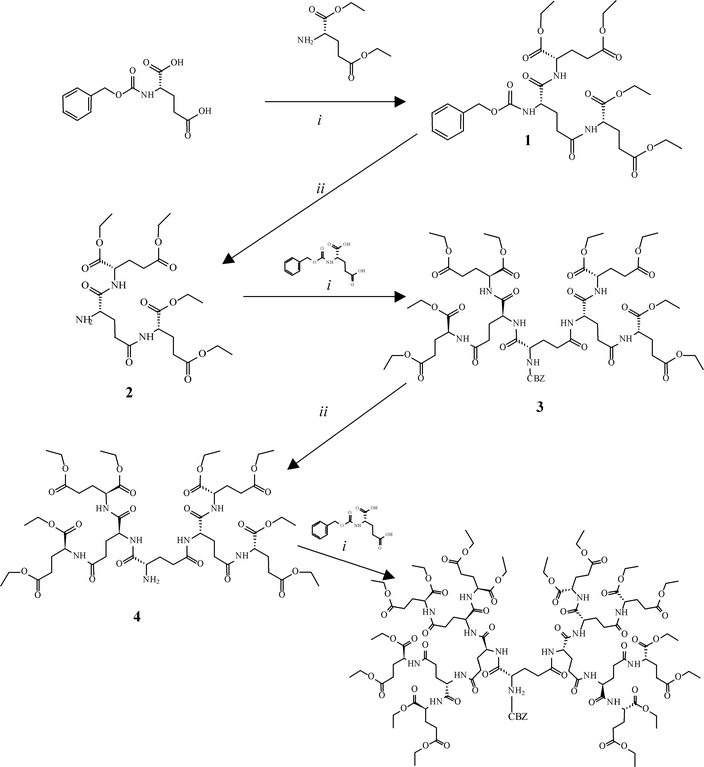
HOW ARE THEY CREATED?The first dendrimer was synthesized by Fritz Vögtle in his paper entitled, “Cascade"- and "Nonskid-Chain-like" Syntheses of Molecular Cavity Topologies” in 1978. Since that period many researchers and companies have followed his direction. Dendrimers can be synthesized into infinite geometries using many types of molecules. They can be tailored for different purposes; however, they are synthesized by two main methods. These methods are divergent and convergent synthesis. |
Divergent synthesis takes place when one molecule is reacted with various reactants. The resulting molecule is then allowed to react to the list of primary reactants and this process continues. In the case of dendrimers, the core molecule generates branches from the primary reactants and the process continues until a sphere is formed. In other words monomer growth diverges from the core. Convergent synthesis is obviously the opposite of divergent synthesis where the branches converge and bond to the core. One can think of the core as being a sticky molecule that other particles stick to. There is a third type of synthesis that uses Diels-Alder reactions or thiol-ene reactions in a process called click chemistry (combine small subunits together).